Background/Purpose: Optimal therapy in childhood onset systemic lupus erythematosus (SLE) lack sufficient data to support clinical decision making. To address this knowledge gap, the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry, a multicenter observational study of childhood onset rheumatic disease, began enrolling individuals with childhood onset SLE and related disorders in 3/2017. Related disorders include Sjogren’s syndrome, mixed connective tissue disease, isolated cutaneous lupus, probable lupus [3 Systemic Lupus International Collaborating Clinics Classification (SLICC) Criteria], and primary antiphospholipid antibody syndrome. We sought to describe the demographics, medication regimens and disease activity indices of this cohort after 33 months of enrollment.
Methods: Inclusion criteria for the cohort include diagnosis of SLE or a related condition within 24 months or new onset of lupus nephritis within 24 months. Data are collected prospectively at 6 month intervals from CARRA sites (n = 70). To date, 704 individuals have been enrolled, 661 with complete data at baseline, 465 at 6 months, 322 at 12 months, 172 at 18 months, 24 at 72 months and 22 at 30 months. Data were summarized with descriptive statistics including means and standard deviations for continuous variables and percentages for categorical variables.
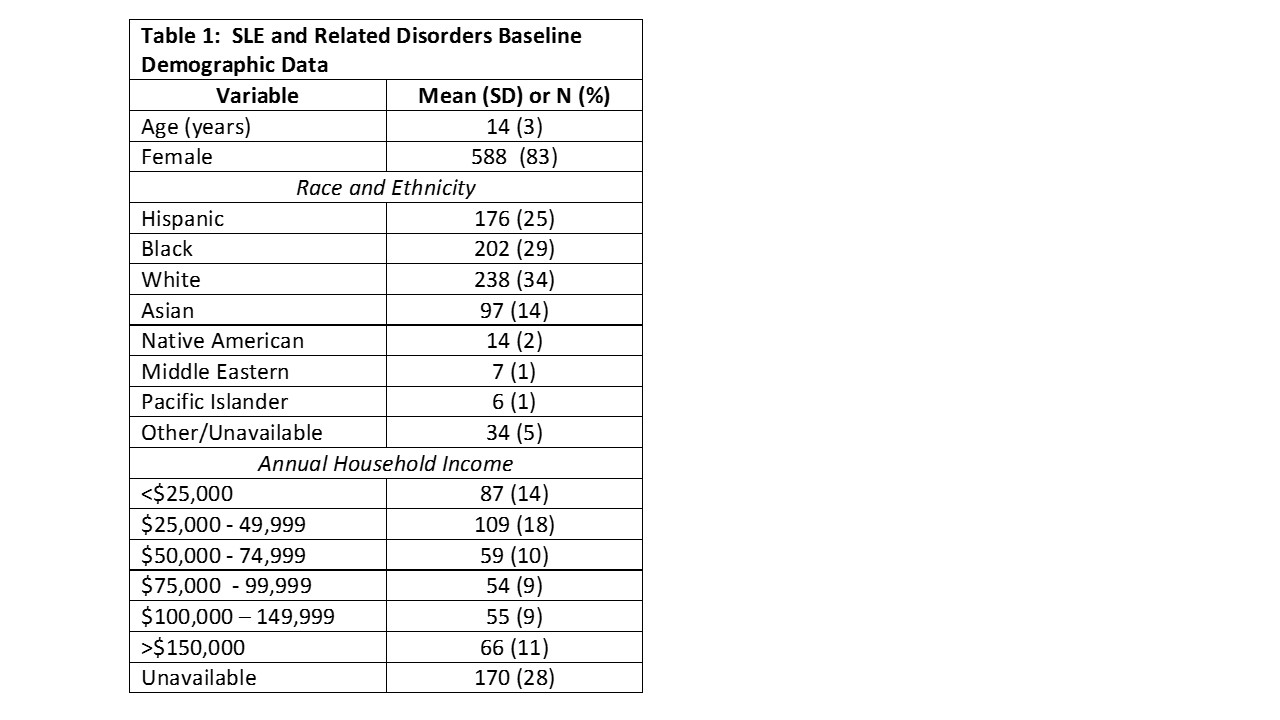
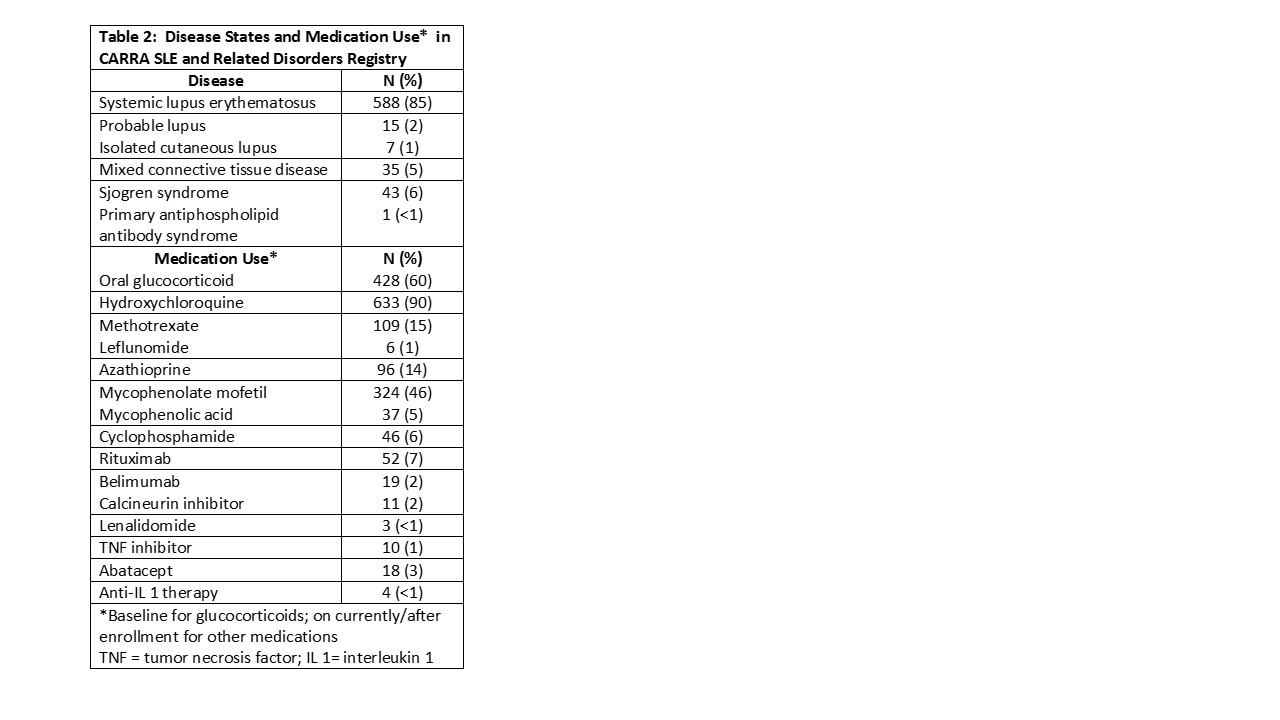
Results: Baseline demographic data are depicted in Table 1. The majority of participants (n=648, 92%) were enrolled at United States (US) sites, 42 (6%) at Canadian sites and 7 (1%) in other countries. Of the females, 440 (75%) were post-menarchal at enrollment. For US participants, 345 (49%) had private insurance, 15 (2%) Medicare and 223 (32%) Medicaid insurance. Half of participants (n=357, 51%) were enrolled within 6 months of diagnosis. SLE was the most common diagnosis (Table 2). The SLE Disease Activity Index 2000 (SLEDAI 2K), completed for 664 participants at baseline, had mean score of 5.8 (6.6). The mean physician assessment of global activity (PGA) score, completed for 605 participants at baseline, was 2.6 (2.3) The SLICC Damage Index, completed in 685, had mean score 0.3 (0.76); 78% had no damage. Mean CHAQ score, completed for 615 participants, was 0.3 (0.6) at baseline. Medication use is detailed in Table 2. The majority (525; 75%) reported ever using glucocorticoids, and 428 (61%) were on steroids at baseline visit. The vast majority were on hydroxychloroquine; mycophenolate mofetil was the most commonly used immunosuppressant.
Conclusion: The CARRA Registry has successfully enrolled > 700 participants with child onset SLE and related conditions over 33 months. The CARRA SLE and related disorders cohort represents a large, diverse patient population with active disease as determined by baseline SLEDAI-2K and PGA scores as well as widespread use of glucocorticoids and other immunosuppressant medications. Individuals in the cohort are treated with a broad spectrum of immunosuppressant medications. The CARRA SLE and related disorders cohort is a rich resource for future investigation of the natural history of SLE, treatment approaches and comparative medication efficacy.
The post The Childhood and Rheumatology Research Alliance Systemic Lupus Erythematosus and Related Disorders Cohort appeared first on ACR Meeting Abstracts.