Background/Purpose: In July 2015, the CARRA Registry was re-established as a multi-center observational study that collects essential data from patients with childhood-onset rheumatic diseases. The primary objective is to evaluate the safety of therapeutic agents. Key secondary objectives are to document the clinical courses and medication treatment patterns of patients and evaluate determinants of clinical outcomes. This abstract describes the clinical characteristics of patients with juvenile idiopathic arthritis (JIA) in the CARRA Registry at the time of enrollment and specifically quantifies the inception cohorts of newly diagnosed JIA and newly prescribed medications.
Methods: The CARRA Registry enrolled patients with JIA from CARRA pediatric rheumatology centers throughout the United States and Canada. Enrollment was open to all categories of JIA. We present enrollment characteristics of JIA patients enrolled prior to 10 December 2019. To facilitate retrospective cohort studies within the Registry, we determined the number of patients newly diagnosed at enrollment (defined as enrollment visit ≤ 6 months after diagnosis), or who initiated a new DMARD and/or biologic at or any time after enrollment. Missing values were ignored.
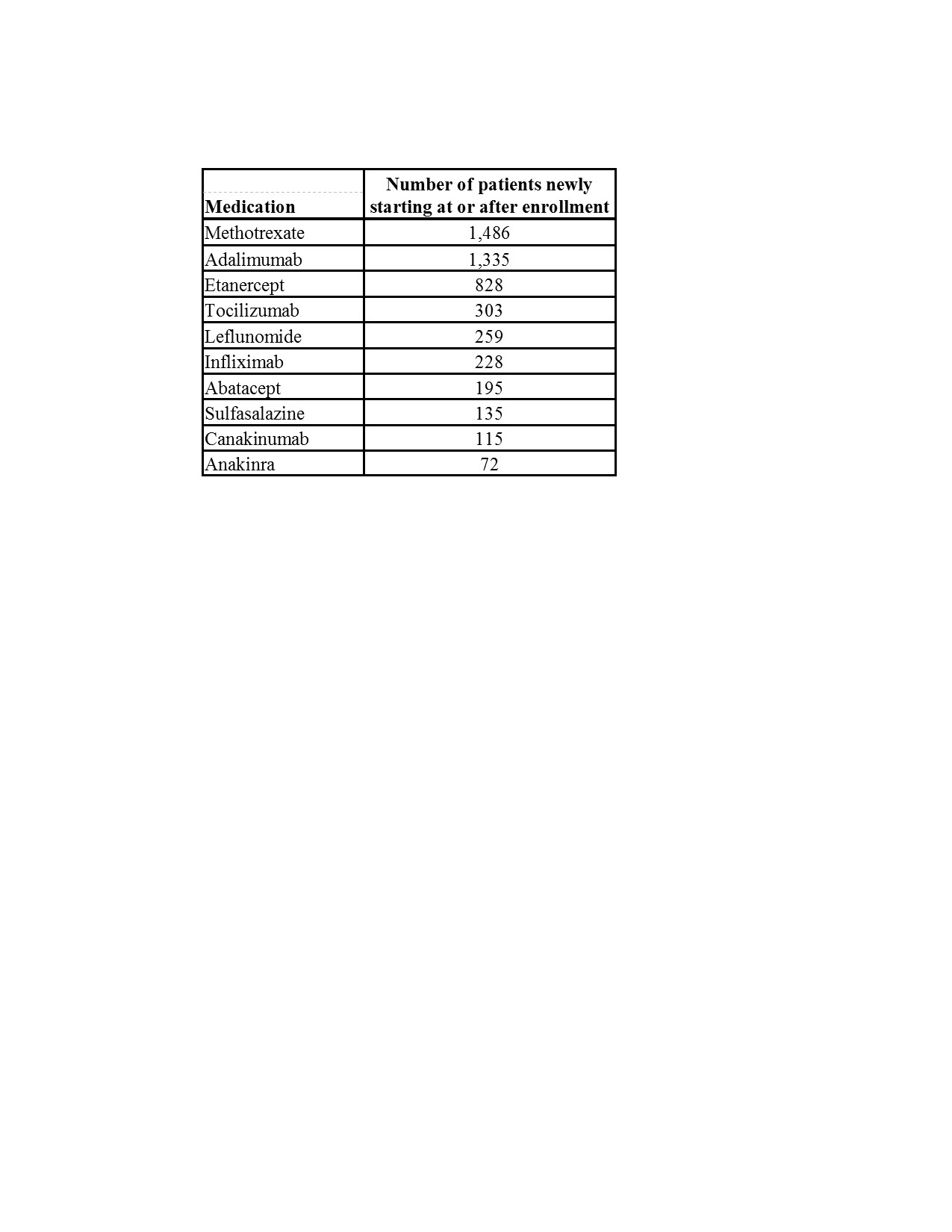
Results: As of 10 December 2019, there were 8,935 patients with JIA enrolled in the CARRA Registry from 70 CARRA centers; 31 centers had enrolled at least 100 patients with JIA. The clinical characteristics of all patients at enrollment are shown in Table 1. A total of 2,577 (29%) patients were enrolled within 6 months of JIA diagnosis. Patients who newly started JIA medications at or after enrollment are represented in Table 2. There were 242 serious adverse events (SAE) reported in 13,348 person-years of observation for overall SAE rate of 1.8 per 100 person-years.
Conclusion: The CARRA Registry has successfully enrolled almost 9,000 patients with JIA in the 4 years since its inception. Over 2,500 patients were enrolled within 6 months of diagnosis, creating a large prospectively followed inception cohort for further study. There are sufficient numbers of new medication initiations among patients enrolled in the Registry to allow investigators to rigorously assess medication effectiveness and safety.
The post Patients with Juvenile Idiopathic Arthritis in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry: Clinical Characteristics and Inception Cohorts appeared first on ACR Meeting Abstracts.